DIAPHORASE from microorganism.
DAD-311
NAD(P)H:(acceptor)oxidoreductase(EC 1.6.99.-)
NAD(P)H+ H⁺+ Acceptor(ox) ► NAD(P) ⁺+ Acceptor(red)
| Appearance: | Yellowish amorphous powder, lyophilized | ||
|---|---|---|---|
| Activity: | Grade III 500 U/mg-solid or more | ||
| Contaminants: | Myokinase ≤5.0×10⁻¹% NADH oxidasee ≤1.0×10⁻¹% |
||
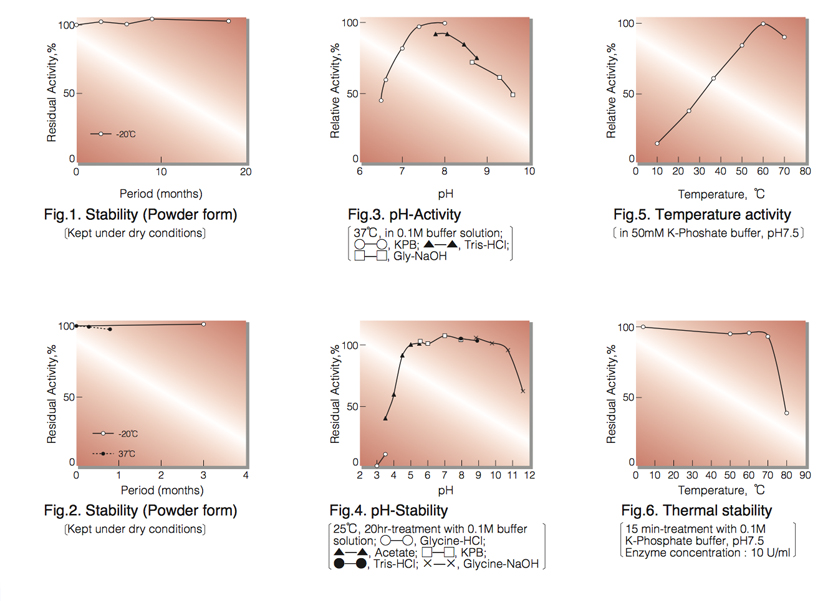
| Stability: | Stable at -20°C (Fig.1) |
|---|---|
| Molecular weight : | approx. 48,000 |
| Michaelis constant: | 2.2 × 10-⁴M (NADH), 2.9 × 10-²M(NADPH) |
| Inhibitors : | Fe³⁺, Mn²⁺, Cu²⁺, Pb²⁺ |
| Isoelectric point: | 5.0 |
| Optimum pH : | 8.0(Fig.3) |
| Optimum temperature : | 60°C(Fig.4) |
| pH Stability : | 5.0–10.0 (25°C, 20hr)(Fig.5) |
| Thermal stability : | below 70°C (pH7.5, 15min)(Fig.6) |
| Substrate specificity: | Either NADH or NADPH can be used as a reductant. |
| Effect of various chemicals : | (Table 1) |
APPLICATIONS
This enzyme is useful for colorimetric determination of NAD(P)H and many dehydrogenases when coupled with various dyes which act as hydrogen acceptors from NAD(P)H.
ASSAY
Principle:
diaphorase
NADH +H⁺+DCPIP ►NAD⁺ + DCPIP(red)
The reduction of DCPIP(2,6-dichlorophenol-indophenol) is measured at 600 nm by spectrophotometry.
Unit definition:
One unit causes the decrease of one micromole of DCPIP per minute under the conditions described below.
Method:
| A. Buffer solution : | 0.2 M Tris-HCl, pH 8.0 |
|---|---|
| B. NADH solution: | 36 mM (Prepare freshly and store on ice) |
| C. DCPIP solution : | 2.4 mM [7.8mg DCPIP(Mw:326.11)/10ml of H₂O](Should be prepared fresh) |
| D. Enzyme diluent: | Buffer solution (A) containing 0.5% of Tween20 |
Procedure
| Concentration in assay mixture | |
|---|---|
| Tris buffer | 27 mM |
| NADH | 1.2 mM |
| DCPIP | 80 µM |
| Tween20 | ca. 167 µg/ml |
1. Prepare the following reaction mixture in a cuvette
(d=1.0cm) and equilibrate at 37oC for about 4 minutes.
2.4ml H₂O
0.3ml Buffer solution (A)
0.1ml NADH solution (B)
2. Add 0.1 ml of the enzyme solution* and mix by gentle pipetting and equilibrate at 37oC for another 1 min.
3. Add 0.1 ml of DCPIP solution (C) and mix by rapid inversion.
4. Record the decrease of optical density at 600 nm against water for 3 to 4 min in a spectrophotometer
thermostated at 37oC, and calculate the ΔOD per minute from the initial linear portion of the curve (OD test).
At the same time , measure the blank rate (OD blank) by the same method as test except that the enzyme
diluent is added instead of the enzyme solution.
* Dissolve the enzyme preparation in ice-cold buffer solution (A) (approx. 1.0% solution), dilute to 0.10−0.25U/ml with ice-cold enzyme diluent (D) and store on ice.
Calculation
Activity can be calculated by using the following formula :

ΔOD/min(OD test–OD blank)× Vt× df
Volume activity (U/ml) = = ΔOD/min× 1.43× df
20.9×1.0×Vs
Weight activity (U/mg)=(U/ml)×1/C
- Vt
- : Total volume (3.0ml)
- Vs
- : Sample volume (0.1ml)
- 20.9
- : Millimolar extinction coefficient of DCPIP under the assay conditions (㎠/micromole)
- 1.0
- : Light path length (cm)
- df
- : Dilution factor
- C
- : Enzyme concentration in dissolution (C mg/ml)
| Chemical | Conc.(mM) | Residual activity(%) |
Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|---|---|---|
| None | − | 100 | NaN₃ | 2.0 | 104 |
| Metal salt | 2.0 | EDTA | 5.0 | 105 | |
| MgCl₂ | 102 | o-Phenanthroline | 2.0 | 105 | |
| CaCl₂ |
99 | α,α′-Dipyridyl | 1.0 | 102 |
|
| Ba(OAc)₂ | 100 |
Borate | 5.0 | 104 | |
| FeCl₃ | 4.4 |
IAA | 2.0 | 105 | |
| CoCl₂ | 94 | NEM | 2.0 | 106 |
|
| MnCl₂ | 55 | Hydroxylamine | 2.0 | 107 | |
| ZnCl₂ | 84 | Triton X-100 | 0.10% | 109 | |
| Cd(OAc)₂ | 101 | Brij 35 | 0.10% | 109 | |
| NiCl₂ | 101 | Tween 20 | 0.10% | 116 | |
| CuSO₄ | 23 | Span 20 | 0.10% | 113 | |
| Pb(OAc)₂ | 46 | Na-cholate | 0.10% | 110 |
|
| AgNO₃ | 94 | SDS | 0.05% | 91 | |
| MIA | 1.0 | 104 |
DAC | 0.05% | 110 |
| NaF | 2.0 | 105 |
MIA, Monoiodoacetate; EDTA, ethylenediaminetetraacetate; IAA, iodoacetamide; NEM, N-Ethylmaleimide; SDS, sodium dodecyl sulfate; DAC, Dimethylbenzylalkylammonium chloride
To get a quote, contact us at info@toyobousa.com, or INQUIRY.