SARCOSINE OXIDASE from Microorganism
SAO-351
Sarcosine:oxygen oxidoreductase (demethylating) (EC 1.5.3.1)
Sarcosine + O₂ + H₂O ► Glycine + Formaldehyde + H₂O₂
| Appearance: | Yellowish amorphous powder, lyophilized |
|---|---|
| Activity: | GradeⅢ 8.0U/mg-solid or more |
| Contaminant: | Catalase ≤1.0% |
| Stabilizers: | Potassium gluconate |
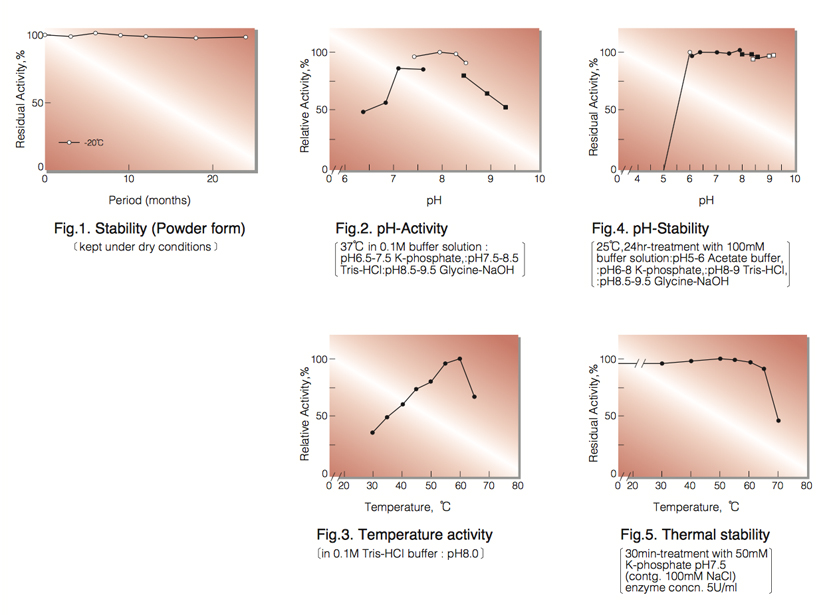
| Stability: | Stable at -20°C for at least One year (Fig.1) |
|---|---|
| Molecular weight : | approx.43,000 (by SDS-PAGE) |
| Isoelectric point : | 4.8±0.1 |
| Michaelis constant: | 2.8×10⁻³M |
| Inhibitors: | Cu⁺⁺, Ag⁺, Hg⁺⁺, p-chloromercuribenzoate, N-ethylmaleimide, SDS |
| Optimum pH : | 7.5-8.5(Fig.2) |
| Optimum temperature : | 55-60°C(Fig.3) |
| pH Stability : | 6.0-9.5 (25°C, 20hr)(Fig.4) |
| Thermal stability : | below 60°C (pH 7.5, 30min)(Fig.5) |
| Effect of various chemicals : | (Table.1) |
APPLICATIONS
This enzyme is useful for enzymatic determination of creatinine, creatine, and sarcosine when coupled with creatinine amidohydrolase (CNH-211, CNH-311) and creatine amidinohydrolase (CRH-211, CRH- 221, CRH-229).
ASSAY
Principle:
sarcosine oxidase
Sarcosine+O₂+H₂O ► Glycine+Formaldehyde+H₂O₂
peroxidase
2H₂O₂+4-Aminoantipyrine+Phenol ► Quinoneimine dye+4H₂O
Unit definition:
One unit causes the formation of one micromole of hydrogen peroxide (half a micromole of quinoneimine dye) per
minute under the conditions described below.
Method:
| A. Sarcosine solution: | 0.2M [Weight 1.78g of sarcosine (MW=89.09), dissolve in 80ml of 0.125M Tris- HCl buffer, pH 8.0 containing 0.125% of Triton X-100 and, after adjusting pH 8.0 at 25°C with 1.0N NaOH or 1.0N HCl, fill up to 100ml with H₂O.](Stable for one week if stored at 0-5°C) |
|---|---|
| B. 4-AA solution: | 0.1% (100mg of 4-aminoantipyrine/100ml of H₂0)(Store at 4°C in a brownish bottle) |
| C. Phenol solution: | 0.1% (100mg of phenol/100ml of H₂O)(Store at 4°C in a brownish bottle) |
| D. Peroxidase solution : |
0.025% [25mg of peroxidase (110 purpurogallin units/mg)/100ml of H₂O](Should be prepared fresh) |
| E. SDS solution: | 0.25% (1.25g of sodium dodecyl sulfate/500ml of H₂O) |
| F. Enzyme diluent: | 20mM Tris-HCl buffer, pH 8.0 containing 2.0mM EDTA |
Procedure
| Concentration in assay mixture | |
|---|---|
| Tris-HCl buffer | 48 mM |
| Sarcosine | 95 mM |
| 4-Aminoantipyrine | 0.47mM |
| Phenol | 2.0 mM |
| Triton X-100 | 0.045 % |
| POD | ca.5.2 U/ml |
1. Prepare the following working solution (100 tests) in a brownish bottle and store on ice.
50ml Sarcosine solution (A)
10ml 4-AA solution (B)
20ml Phenol solution (C)
20ml Peroxidase solution (D)
2. Pipette 1.0ml of working soluton into a test tube and equilibrate at 37°C for about 5 minutes.
3. Add 0.05ml of the enzyme solution*and mix.
4. After exactly 10 minutes at 37°C, add 2.0ml of SDS solution (E) to stop the reaction and measure the optical
density at 500nm against water (OD test).
At the same time, prepare the blank by using the same method as the test except that the enzyme diluent is used instead of the enzyme solution (OD blank).
* Dissolve the enzyme preparation in ice-cold enzyme diluent (F) and dilute to 0.07-0.17U/ml with the same buffer, immediately before assay.
Calculation
Activity can be calculated by using the following formula :

ΔOD (OD test-OD blank)×Vt×df
Volume activity (U/ml) = = ΔOD×0.917×df
13.3×1/2×1.0× t ×Vs
Weight activity (U/mg)=(U/ml)×1/C
- Vt
- : Total volume (3.05ml)
- Vs
- : Sample volume (0.05ml)
- 13.3
- : Millimolar extinction coefficient of quinoneimine dye under the assay condition (㎠/micromole)
- 1/2
- : Factor based on the fact that one mole of H₂O₂ produced half a mole of
quinoneimine dye - t
- : Reaction time (10 minutes)
- 1.0
- : Light path length (cm)
- C
- : Enzyme concentration in dissolution (c mg/ml)
REFERENCES
- N.Mori, M.Sato, Y.Tani and Y.Yamada; Agric.Biol.Chem., 44, 1391 (1980).
- M.Suzuki; J. Biochem., 89, 599 (1981).
- M.Suzuki and M.Yoshida; Proceedings of the Symposium on Chemical Physiolosy and Pathology (Kyoto), Vol16, p.220(1976).
- T.Kinoshita and Y.Hiraga; Chem.Pharm.Bull., 28, 3501 (1980).
- Y.Nishiya, S.Zuihara and T.Imanaka; APPLIED AND ENVIROMENTAL MICROBIOLOGY., 61, 367 (1995).
| Chemical | Concn.(mM) | Residual activity(%) |
Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|---|---|---|
| None | − | 100 | NaF | 2.0 | 99 |
| Metal salt | 2.0 | NaN₃ | 20.0 | 90 | |
| MgCl₂ |
99 | EDTA | 5.0 | 97 | |
| CaCl₂ | 99 | o-Phenanthroline | 2.0 | 99 | |
| Ba(OAc)₂ | 98 | α,α′-Dipyridyl | 2.0 | 97 | |
| FeCl₃ | 99 | Borate | 50.0 | 98 | |
| CoCl₂ | 98 | IAA | 2.0 | 97 | |
| MnCl₂ | 99 | NEM | 2.0 | 74 | |
| Zn(OAc)₂ | 98 | Hydroxylamine | 2.0 | 97 | |
| Cd(OAc)₂ | 99 | Triton X-100 | 0.10% | 99 | |
| NiCl₂ | 96 | Brij 35 | 0.10% | 99 | |
| CuSO₄ | 43 | Tween 20 | 0.10% | 97 | |
| Pb(OAc)₂ | 98 | Span 20 | 0.10% | 101 | |
| AgNO₃ | 0.4 | Na-cholate | 0.10% | 99 | |
| HgCl₂ | 0.4 | SDS | 0.05% | 68 | |
| PCMB | 2.0 | 36 | DAC | 0.05% | 97 |
| MIA | 2.0 | 101 |
Ac, CH₃CO; PCMB; p-Chloromercuribenzoate, MI; Monoiodoacetate, EDTA; Ethylenediaminetetraacetate, IAA; Iodoacetate, NEM; N-Ethylmaleimide, SDS; Sodium dodecyl sulfate, DAC; Dimethylbenzylalkylammonium chloride.
To get a quote, contact us at info@toyobousa.com, or INQUIRY.