GLUCOSE-6-PHOSPHATE DEHYDROGENASE from Leuconostoc mesenteroides
| Appearance: | White amorphous powder, lyophilized | ||
|---|---|---|---|
| Activity: | GradeⅢ 400U/mg-solid or more (NAD⁺) |
||
| Contaminants: | Creatine phosphokinase ≤1×10⁻³% Phosphoglucomutase ≤1×10⁻³% 6-Phosphogluconate dehydrogenase ≤5×10⁻³% Phosphoglucose isomerase ≤1×10⁻²% Glutathione reductase ≤1×10⁻³% Hexokinase ≤1×10⁻²% Myokinase ≤1×10⁻²% NADH oxidase ≤1×10⁻²% NADPH oxidase ≤1×10⁻²% |
||
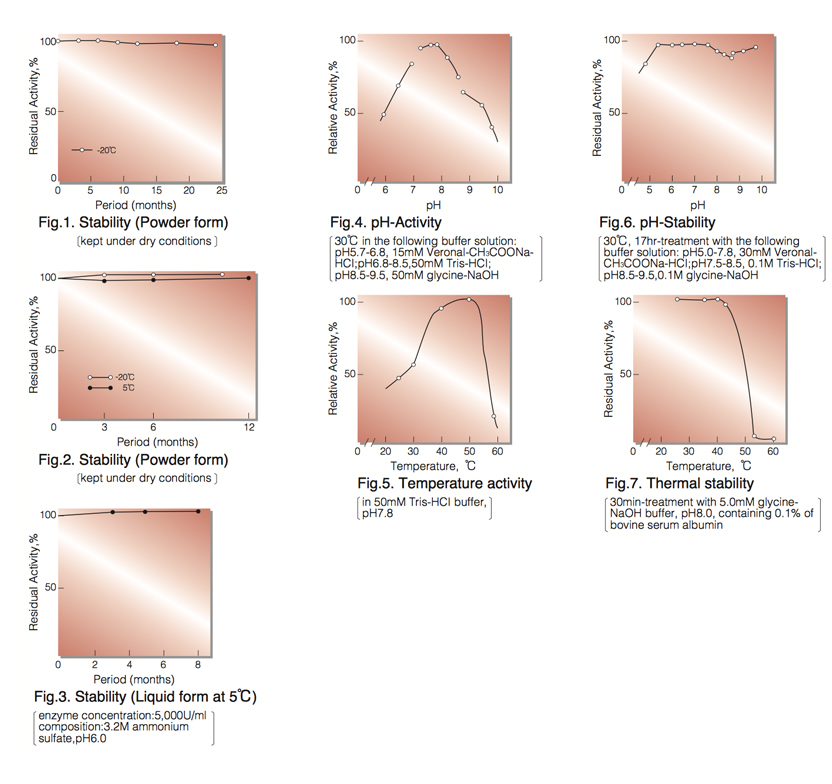
| Stability: | Stable at -20°C for at least one year(Fig.1) |
|---|---|
| Stable at 5°C for at least 6 months (liquid form)(Fig.3) | |
| Molecular weight: | 104,000(two subunits of approx. 55,000)¹ ² ⁾ |
| Isoelectric point: | 4.6 ² ⁾ |
| Michaelis constants ² ⁾: | NAD⁺ linked :1.06×10⁻⁴M (NAD⁺), 5.27×10⁻⁵M (G-6-P) NADP⁺ linked :5.69×10⁻⁶M (NADP⁺), 8.1×10⁻⁵M (G-6-P) |
| Structure: | Neither cysteine nor cystine residues is present in the enzyme molecule¹⁾ and essential lysine is indicated to be at active site.³ ⁾ |
| Inhibitors: | Acyl-CoA,⁴⁾ ATP ⁴⁾ mental ions etc. (Table 1) |
| Optimum pH: | 7.8(Fig.4) |
| Optimum temperature: | 50°(Fig.5) |
| pH Stability: | pH 5.5-7.5 (30°C, 17hr)(Fig.6) |
| Thermal stability: | below 37°C (pH 8.0, 30min)(Fig.7) |
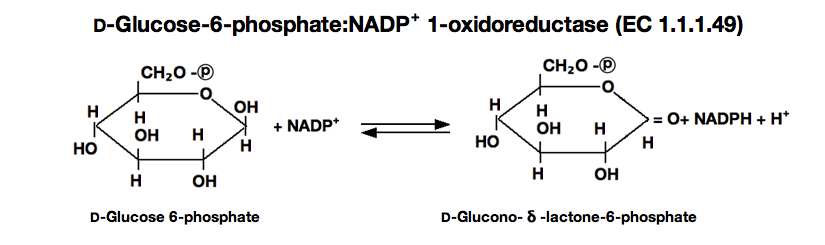
| Substrate specificty: | Either NAD⁺ or NADP⁺ serves as coenzyme, the reaction velocity with NAD⁺ being approximately 1.8 times greater than with NADP.⁺⁵⁾D- Glucose-6-phosphate is a preferential substrate for the enzyme, although D-glucose reacts slowly.⁶⁾Fructose-6-phosphate, fructose- 1, 6-diphoshate and ribose-5-phosphate are not considered to be substrates.⁷⁾ |
APPLICATIONS
This enzyme is useful for enzymatic determation of NAD⁺(NADP⁺) and G-6-P, and activities of phosphoglucose isomerase, phosphoglucomutase and hexokinase. This enzyme is also used for enzymatic determination of glucose when coupled with hexokinase (HXK-311).
G6D-311
ASSAY
Principle:
glucose-6-phophate dehydrogenase(G-6-PDH)
D-Glucose-6-phosphate(G-6-P)+NAD⁺ ►
D-Glucono-δ-lactone-6-phosphate+NADH+H⁺
The appearance of NADH is measured at 340nm by spectrophotometry.
Unit definition:
One unit causes the formation of one micromole of NADH per minute under the conditions described below.
Method:
| A. Tris-HCl buffer, pH 7.8: | 55mM (contaning 3.3mM magnesium chloride) |
|---|---|
| B. NAD⁺ solution: | 60mM (Should be prepared fresh) |
| C. G-6-P solution: | 0.1M glucose-6-phosphate (Should be prepared fresh) |
| D. Enzyme diluent: | 5mM Tris-HCl buffer, pH 7.5, containing 0.1% of bovine serum albumin. |
Procedure
| Concentration in assay mixture | |
|---|---|
| Tris-HCl buffer | 50 mM |
| G-6-P | 3.3 mM |
| NAD⁺ | 2.0 mM |
| MgCl₂ |
3.0 mM |
| BSA | 33µg/ml |
1. Prepare the following reaction mixture in a cuvette (d=1.0cm) and equilibrate at 30°C for about 5 minutes.
2.7 ml Tris-HCl buffer, pH 7.8 (A)
0.1 ml NAD⁺ solution (B)
0.1 ml G・6・P solution (C)
2. Add 0.1ml of the enzyme solution *and mix by gentle inversion.
3. Record the increase in optical density at 340nm against water for 4 to 5 minutes in a spectrophotometer
thermostated at 30°C and calculate the ΔOD per minute from the initial linear portion of the curve (ΔOD test).
At the same time, measure the blank rate (ΔOD blank) by using the same method as the test except that the enzyme diluent is added instead of the enzyme solution.
* Dissolve the enzyme preparation in ice-cold enzyme diluent (D) and dilute to 0.05-0.20U/ml with the same buffer, immediately before the assay.
Calculation
Activity can be calculated by using the following formula :

ΔOD/min (ΔOD test−ΔOD blank ) ×Vt × df
Volume activity (U/ml) = =ΔOD/min×4.82×df
6.22×1.0×Vs
Weight activity (U/mg)=(U/ml)×1/C
- Vt
- : Total volume (3.0ml)
- Vs
- : Sample volume (0.1ml)
- 6.22
- : Millimolar extinction coefficient of NADH (㎠/micromole)
- 1.0
- : Light path length (cm)
- df
- : Dilution factor
- C
- : Enzyme concentration in dissolution (c mg/ml)
REFERENCES
- A.Ishaque,M.Mihausen and H.R.Levy; Biochem. Biophys. Res. Comm., 59, 894 (1974).
- C. Olive, M.E. Geroch and H.R.Levy; J.Biol.Chem., 246, 2043 (1971).
- M.Milhausen and H.R. Levy; Eur.J.Biochem., 50, 453 (1975).
- E.L.Coe and L.-H.Hsu; Biochem. Biophys. Res. Comm., 53, 66 (1973).
- C.Olive and H.R. Levy; Biochem., 6, 730 (1967).
- R.P.Metzger, S.A. Metzger and R.L. Parsons; Arch Biochem. Biophys., 149, 102 (1972).
- Methods in Enzymology, Vol, 1, p328 (S.P.Colowick and N.O.Kapalan,eds.), Academic Press, New York (1955).
| Chemical | Concn.(mM) | Residual activity(%) |
Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|---|---|---|
| None | − | 100 | NEM | 2.0 | 91 |
| Metal salt | 2.0 | PCMB | 2.0 | 96 | |
| AgNO₃ |
86 | MIA |
2.0 | 14 |
|
| Ba(OAc)₂ | 51 | Iodoacetamide | 2.0 | 0 | |
| CaCl₂ | 90 | EDTA | 5.0 | 94 | |
| Cd(OAc)₂ | 74 | (NH₄)₂SO₄ | 20.0 | 98 | |
| CoCl₂ | 80 | Borate | 20.0 | 95 | |
| CuSO₄ | 66 |
o-Phenanthroline | 2.0 | 93 | |
| FeCl₃ | 0 |
α,α′-Dipyridy | 2.0 | 95 | |
| FeSO₄ | 1 | Urea | 2.0 | 93 | |
| HgCl₂ | 84 | Guanidine | 2.0 | 93 | |
| MgCl₂ | 90 | Hydroxylamine | 2.0 | 91 | |
| MnCl₂ | 89 | Na-cholate | 1.0% | 102 | |
| NiCl₂ | 89 | Triton X-100 | 1.0% | 100 | |
| Pb(OAc)₂ |
3 | Brij 35 | 1.0% | 4 | |
| Zn(OAc)₂ | 67 |
SDS | 0.1% | 0 | |
| ZnSO₄ | 53 | Tween 20 | 0.1% | 101 | |
| KF |
2.0 | 93 | Span 20 | 0.1% | 99 |
| NaF |
20.0 | 98 | DAC | 0.1% | 0 |
| NaN₃ | 20.0 | 93 |
Ac, CH₃CO; NEM, N-Ethylmaleimide; PCMB, p-Chloromercuribenzoate; MIA, Monoiodoacetate; EDTA, Ethylenediaminetetraacetate; SDS, Sodium dodecyl sulfate; DAC, Dimethylbenzylalkylammoniumchloride.
To get a quote, contact us at info@toyobousa.com, or INQUIRY.