CATALASE from Microorganism
CAO-519
Hydrogen-peroxide: hydrogen-peroxide oxidoreductase (EC 1. 11. 1. 6)
2H₂O₂ ⇆ O₂ + 2H₂O
| Appearance: | Orive green solution | |
|---|---|---|
| Activity: | Grade V 150,000 U/ml or more | |
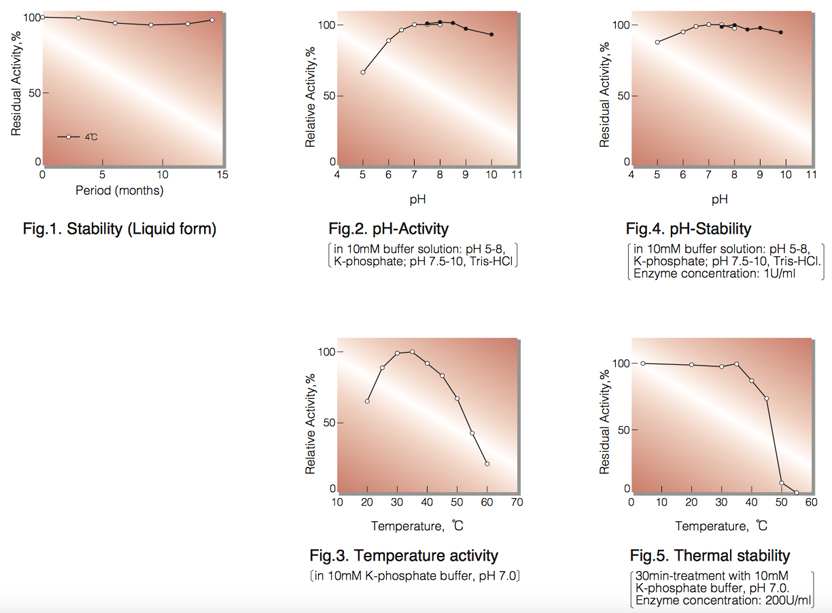
| Stability: | Stable at 4°C (Fig.1) |
|---|---|
| Molecular weight: | approx. 53,000 (by mass spectrometry) |
| Inhibitors : | NaN₃ |
| Optimum pH : | 7.2-9.0(Fig.2) |
| Optimum temperature | 35-40°C(Fig.3) |
| pH Stability |
pH 6.2-8.9 (25°C, 16hr)(Fig.4) |
| Thermal stability : | below 35°C (pH 7.0, 30min)(Fig.5) |
| Effect of various chemicals | (Table 1) |
APPLICATIONS
This enzyme is useful for eliminating the interference of hydrogen-peroxide in clinical analysis.
ASSAY
Principle:
Catalase
2H₂O₂ ►O₂+2H₂O
The disappearance of hydrogen peroxide is measured by the Titanium color method ¹).
Unit definition:
One unit causes the hydrolysis of one micromole of hydrogen peroxide per minute under the conditions described below.
Method:
| A. 10mM Phosphate buffer, pH 7.0 (at 25°C): | |
|---|---|
| B. H₂O₂ solution: | 16mM [0.182ml of 30% (W/V)H₂O₂/100ml of buffer A] (Should be prepare fresh and store on ice.) |
| C. Titanium reagent: | (Nacalai tesque) |
| D. Enzyme diluent: | Buffer A |
Procedure
1. Prepare 0.25ml of the substrate solution (B) into a test tube and equilibrate at 25°C for about 5 minutes
2. Add 0.25ml of the enzyme solution* and mix.
3.After exactly 5 minutes at 25°C, add 2.5ml of Titanium reagent (C) to stop the reaction and
measure the optical density at 410nm against water (OD test)
At the same time, prepare the blank by first mixing the substrate solution with 2.5ml of
Titanium reagent after 5 min-incubation at 25°C, follow by the addition of enzyme solution (OD blank)
* Dilute the enzyme preparation to 0.35-1.35U/ml with ice-cold enzyme diluent (D).
Calculation
Activity can be calculated by using the following formula :

∆OD(OD blank–OD test)×Vt×df
Volume activity (U/ml) = =∆OD×3.43×1/F×df
F×t×1.0×Vs
Weight activity (U/mg) = (U/ml)×1/C
- Vt
- : Total volume (3.0ml)
- Vs
- : Sample volume (0.25ml)
- F
- : Extinction coefficient of Titanium color product developed by the presence of 1.0mM hydrogen peroxide (F should be determined in each lot of Titanium reagent by using a known concentration of hydrogen peroxide. F is usually around 0.7.)
- t
- : Reaction time (5 minutes)
- 1.0
- : Light path length (cm)
- df
- : Dilution factor
- C
- : Enzyme concentration in dissolution (c mg/ml)
REFERENCES
- F.Patti and P.B.-Maury; Bull.Soc.Chem.Biol.,35, 1177 (1953)
| Chemical | Conc.(mM) | Residual activity(%) |
Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|---|---|---|
| None | − | 100 | NaF | 2.0 | 89.3 |
| Metal salt | 2.0 | NaN₃ | 2.0 | 3.8 | |
| AgNO₃ | 76.6 | EDTA | 5.0 | 96.6 | |
| BaCl₂ | 99.6 | IAA | 2.0 | 99.4 | |
| CaCl₂ | 62.4 | Borate | 20 | 98.8 | |
| CoCl₂ | 101.2 | SDS | 0.05% | 96.1 | |
| CuSO₄ | 99.7 | Triton X-100 | 0.10% | 95.7 | |
| FeSO₄ | 98.2 | Brij 35 |
0.10% | 97.8 | |
| MgSO₄ | 99.2 | Span 20 | 0.10% | 94.5 | |
| MnCl₂ | 28.7 | Na-cholate | 0.10% | 95.3 | |
| NiCl₂ | 53.6 | ||||
| ZnCl₂ | 96.1 |
EDTA, ethylenediaminetetraacetate; IAA, iodoacetamide; SDS, sodium dodecyl sulfate;
DAC, Dimethylbenzylalkylammonium chloride
To get a quote, contact us at info@toyobousa.com, or INQUIRY.